MARICOPA COUNTY, Ariz. — It will still be a long time before Arizona residents can expect to return to a pre-COVID world.
The general population will not have the opportunity to get vaccinated until at least April 2021, according to a recent Maricopa County Board of Supervisors meeting.
The meeting Monday outlined the county's plan for distributing the vaccine once it arrives in the state in the coming weeks.
Here are the main takeaways you should know from the meeting:
Herd immunity will not be achieved until summer 2021
Herd immunity, also known as population immunity, is a concept used to represent the idea of a population being protected from a virus if enough of the population become vaccinated.
The World Health Organization (WHO) has explained that vaccinations are the only sure way to obtain herd immunity.
The question of herd immunity was brought up to the Executive Director of the Maricopa County Department of Public Health, Marcy Flanagan, at the board meeting.
"We don't believe herd immunity is going to be reached until...at the earliest, spring. More likely, it will be the summer of 2021," Flanagan said.
Certain groups will be prioritized for the vaccine and the general public will not able to get vaccinated until later next year.
Who will be getting the vaccine first, second, and third?
Flanagan also laid out the predicted schedule of who will be prioritized to receive the vaccine and when they can expect to receive it. The county's plan exists in three phases.
The state expects to receive around 60,000 doses, with Maricopa County receiving 40,000 immediately after FDA approval later this week.
The state and individual counties decide who would receive the vaccine first. The people in the prioritized groups in each phase will be assessed on how high risk they are based on a variety of metrics, including risk of exposure, economic impact of the loss of work and availability to telework.
Phase One (December 2020 to Spring 2021):
This will take place right when the state receives the first batch of vaccines. Flanagan clarified that the vaccine is expected to come through at a "slow drip" speed, as the CDC has recommended that officials make plans assuming there will not be enough to go around at first.
The expected 40,000 doses will be distributed to two of the five regional points of dispense (POD).

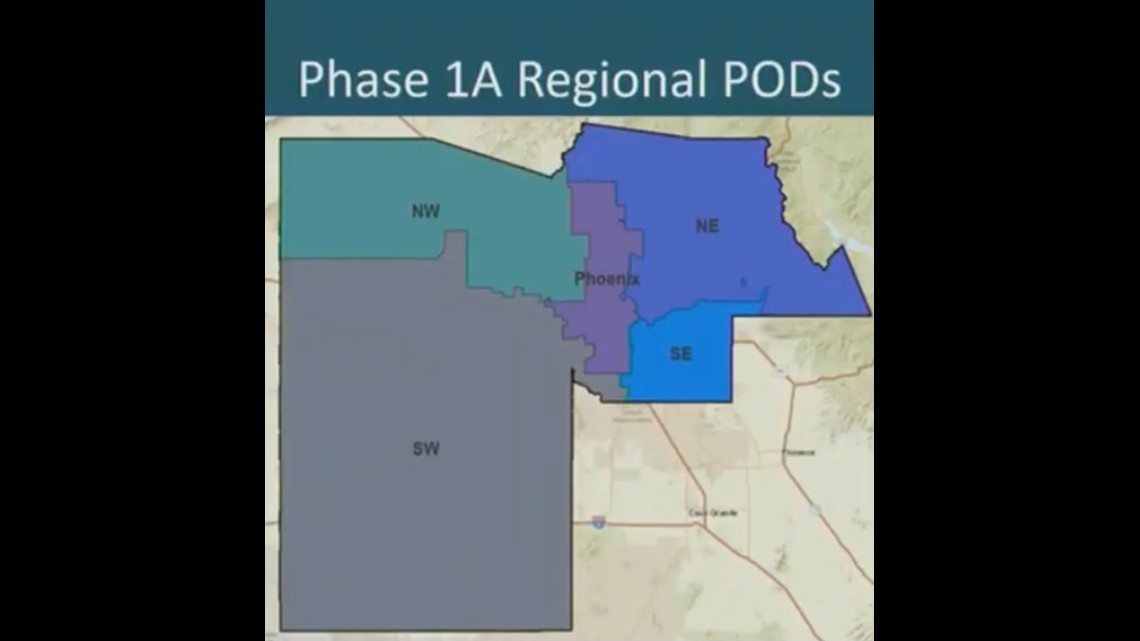

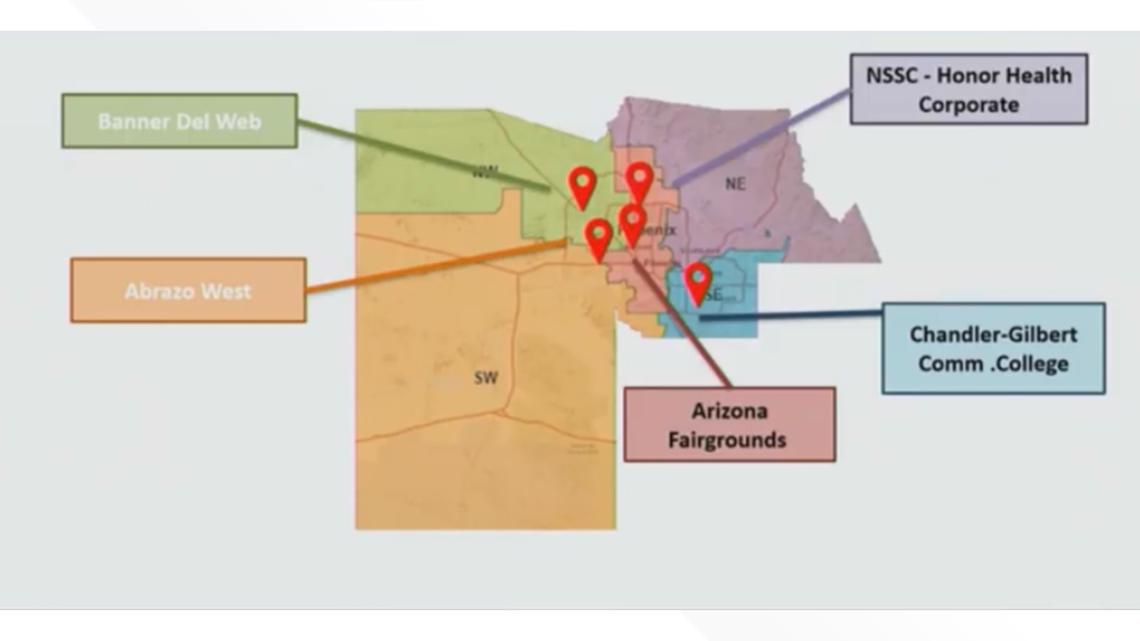
The expected 40,000 doses will be distributed to Banner Del Webb and Honor Health Corporate, two of the five regional points of dispense (POD).
Arizona is expected to receive 200,000 doses this month, Flanagan said. Pharmacies and tribes will get some of the initial batch of doses with the goal of immunizing long-term care facility staff in critical areas.
Phase 1a, which will happen between December 2020 through early 2021, will have vaccinations ready for healthcare personnel, emergency medical system (EMS) workers, and long-term care, assisted living, and skilled nursing staff and residents.
Phase 1b and 1c, from early 2021 to Spring 2021, will offer the rest of the vaccinations to remaining healthcare workers, high-risk individuals above the age of 65, and other essential workers including teachers, law enforcement, and childcare workers.
Phase Two (Spring 2021 to Summer 2021):
It will be in early spring of next year when there should be enough of the supply of the vaccines to meet demand. This is when vaccinations can start being offered beyond the initial priority populations.
Here, the remainder of the priority groups will receive the vaccine, if necessary, along with critical workers in other fields and the general public.
Phase Three (Summer 2021 and beyond):
This is when the vaccination rollout plan will shift over to a routine strategy, and a sufficient supply of the vaccines to meet public demand will still be in place.
The same populations will be allowed to get the vaccine here as in Phase Two, including anyone still needed to get the vaccine from Phase 1, critical workers, and the general population.
Is the vaccine safe?
Both Pfizer and Moderna are mRNA (messenger ribonucleic acid), which mean they do not contain the complete virus. They contain a segment of the viral genome that generate an immune response as if the body was fighting the live virus, which results in antibodies without becoming infected.
These vaccines are unlike they place a weakened or inactivated germ into our bodies.
mRNA vaccines are teachers, instructing our cells on how to make the protein which triggers our immune systems to produce antibodies and protect us from getting infected when COVID-19 enters our bodies.
Facts about the COVID-19 mRNA vaccines
The vaccine CANNOT give someone COVID-19 and do not affect or interact with our DNA in anyway.
Health experts do expect side effects due to the vaccine as seen in others, such as local inflammation at the injection site or soreness and systematic inflammation producing a fever, muscle aches or headache. The stronger a person's immune system, the more likely there will be side effects. The healthier the individual, the stronger the immune system.
What about outreach?
During the presentation, Supervisor Steve Gallardo asked about educating and outreach to communities that may have cultural differences and language barriers, especially with non-medical frontline workers.
Executive Director Marcy Flanagan pointed out there was a lack of minority representation during the vaccine trials and that after FDA final approval, local health experts will review data and work with strategic messaging.
"There are going to be sub populations and sub groups that are more hesitant to receive the vaccine. We will take special care to get the right partners involved to deliver that messaging," said Flanagan.
You can see more tips when it comes to social distancing and being COVID-19 safe on our YouTube playlist here.